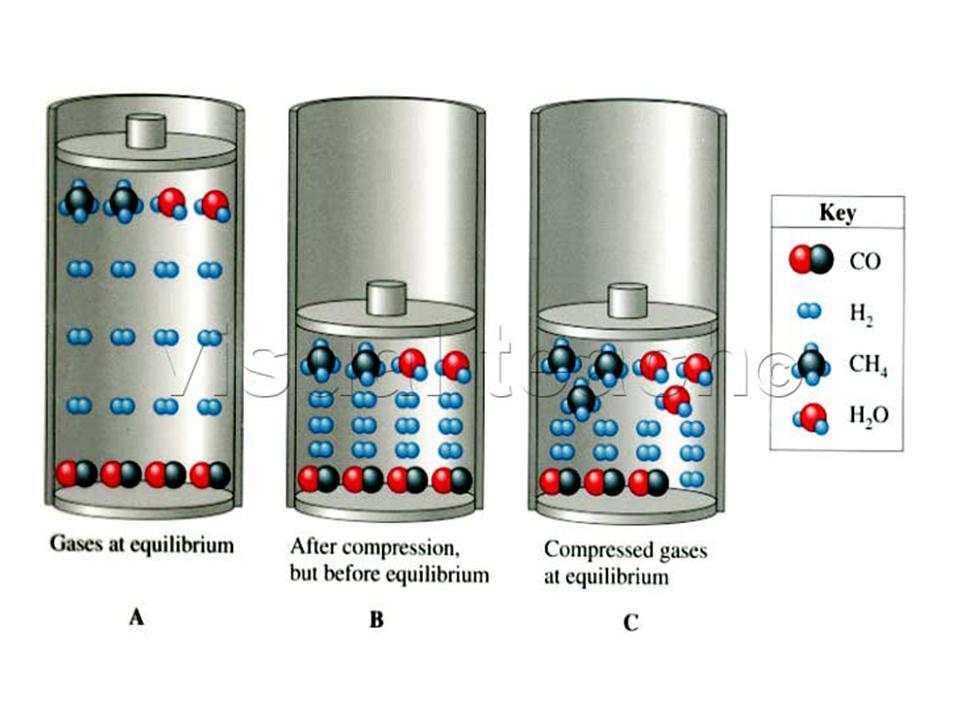
Description: Gases -- Equilibrium vs. Pressure
Image copyright: http://www.ttevisual.com/chemistry/images/4.41.2-(eb14.9).jpg
In a closed system, matter cannot enter or leave, but energy can. For example, energy can be added to a closed system in the form of heat or ice can be added to cause energy to leave a system.
Equilibrium is a very important chemical concept and we will study its relationship to changes of state (i.e. -- liquid, gas, or solid).
This page was
edited
by Dawn White (Teacher: Dawn White) using
Web Poster Wizard.